Tao Dong
Co-Director CAMS Oxford Institute
- Ita Askonas Professor of Translational Immunology
- Founding Director (Oxford), CAMS-Oxford joint International Centre for Translational Immunology
- Founding Director (Oxford), Chinese Academy of Medical Sciences Oxford Institute (COI)
Biography
Tao Dong is the founding director of the CAMS-Oxford Institute based in Nuffield Department of Medicine, Oxford University since 2019. She has held the post of Professor of Immunology in the MRC Human Immunology Unit at Oxford University since 2014 and is a Senior Fellow at University College Oxford. In May 2023, Tao was appointed the Ita Askonas Professorship of Translational Immunology.
Tao moved to Oxford University in 1993 where she received a DPhil degree in Immunology in 1998. In 2010 she became the Head of the human anti-viral and anti-cancer cytotoxic T cell laboratory and subsequently Program Leader in the MRC Human Immunology Unit at Oxford University. She has served as a panel member in varies international funding organisations, and SAB members for several pharmaceutical companies.
Research Interests
The aim of Tao Dong’s research group is to investigate the functional aspects of antigen specific cytotoxic T cells (CTL) with a focus on the factors affecting CTL in controlling virus infection and cancer progression. While a robust and appropriate T cell response is typically beneficial to the host during human infections, a weak or inappropriate response can be ineffective or even have a detrimental effect. Over the past two decades, they have been working to understand the key factors required for efficient viral control by T cells in a number of different viral infections and cancer. Establishing both ex-vivo and in-vitro T cell functional evaluation platforms for antigen-specific T cells isolated from tissue and blood. By linking functional data with multi-omic single cell and T cell receptor (TCR) repertoire analysis, they continue to identify potential targets and pathways to augment and control the immune response as a way of improving the outcome of several important human diseases including SARS-CoV-2 virus infection and cancer.
Key Publications
Unconventional human CD61 pairing with CD103 promotes TCR signaling and antigen-specific T cell cytotoxicity
Abd Hamid M. et al. (2024) Nature Immunology
Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19
Peng Y. et al. (2020), Nature Immunology
An immunodominant NP105–113-B*07:02 cytotoxic T cell response controls viral replication and is associated with less severe COVID-19 disease
Peng Y. et al. (2022), Nature Immunology
Long-persisting SARS-CoV-2 spike-specific CD4+ T cells associated with mild disease and increased cytotoxicity post COVID-19
Liu G. et al. (2025), Nature Communications, 16
T cell memory response to MPXV infection exhibits greater effector function and migratory potential compared to MVA-BN vaccination
Chen J-L. et al, (2025), Nature Communications, 16
Tumor-specific CD8 T cell characterization in HR+ breast cancer reveals an impaired antitumoral response in patients with lymph node metastasis
Pinho MP. et al, (2025), Cell Reports Medicine, 102252 - 102252
Vaccine-induced CD8+ T cells are key to protection from SARS-CoV-2
Antoun E. et al. (2023), Nature Immunology
Enriched HLA-E and CD94/NKG2A Interaction Limits Antitumor CD8+ Tumor-Infiltrating T Lymphocyte Responses
Abd Hamid M. et al. (2019), Cancer Immunology Research
Interferon-induced transmembrane protein-3 genetic variant rs12252-C is associated with severe influenza in Chinese individuals
Zhang YH. et al. (2013), Nature Communications, 4
Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals
Lee LY-H. et al. (2008). Journal of Clinical Investigation.
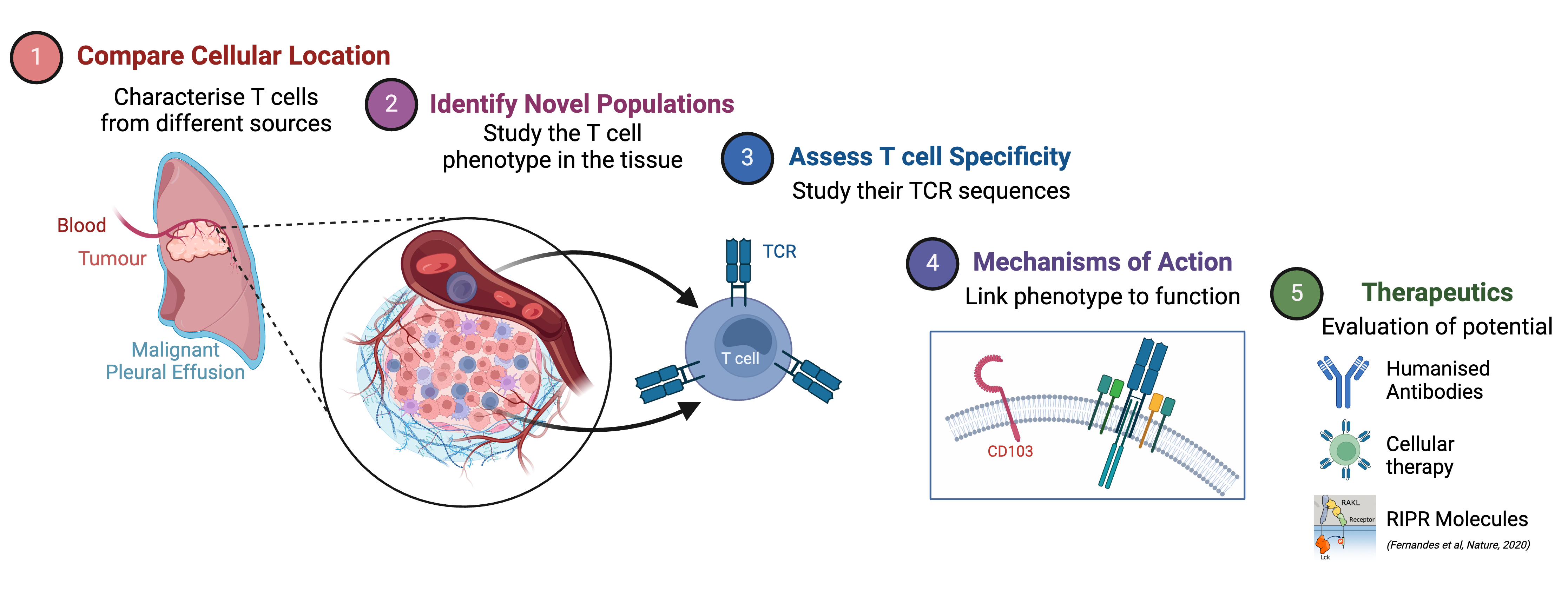
Understanding the role of T cells in cancer
Our group are working to characterise and understand the T cell repertoire present in multiple sites that are influenced by cancer. By comparing T cells from different locations we can identify location-specific differences and potentially identify novel populations of T cells that are specific to a particular cancer. Once these T cells have been identified, we can assess their specificity by looking at their T cell receptor (TCR) sequences. Finally, we can investigate mechanisms of action and link phenotypic data to function, potentially opening avenues for therapeutic intervention in the future.
Understanding the role of T cells in emerging viral infections

For many years, our lab has been able to identify and characterise virus-specific T cells. During the pandemic we were able to streamline this process and create a pipeline for analysis of virus-specific T cells throughout the course of infection. Starting with identification of viral antigens through peptide screens, we can then identify key responses or factors that are associated with disease; establishing those that are protective from those that are pathogenic. With our platform we are able to evaluate how the long-term memory response is altered over time, as well as following re-infection or exposure to vaccines. Finally, we can rapidly assess how viral variants affect T cell responses. Altogether this allows us to get a good understanding of T cell responses in emerging viral infections and provides key research for future vaccines.
Recent publications
-
Pleural fluid proteomics from patients with pleural infection shows signatures of diverse neutrophilic responses: The Oxford Pleural Infection Endotyping Study (TORPIDS-2)
Journal article
Kanellakis NI. et al, (2025), European Respiratory Journal, 66, 2500010 - 2500010
-
Tumor-specific CD8 T cell characterization in HR+ breast cancer reveals an impaired antitumoral response in patients with lymph node metastasis
Journal article
Pinho MP. et al, (2025), Cell Reports Medicine, 102252 - 102252
-
Immunogenicity of MVA-BN vaccine deployed as mpox prophylaxis: a prospective, single-centre, cohort study and analysis of transcriptomic predictors of response
Journal article
Drennan PG. et al, (2025), The Lancet Microbe, 6, 101045 - 101045
-
T cell memory response to MPXV infection exhibits greater effector function and migratory potential compared to MVA-BN vaccination
Journal article
Chen J-L. et al, (2025), Nature Communications, 16
-
Incidence and prevalence of asthma, chronic obstructive pulmonary disease and interstitial lung disease between 2004 and 2023: harmonised analyses of longitudinal cohorts across England, Wales, South-East Scotland and Northern Ireland.
Journal article
Whittaker H. et al, (2025), Thorax